Introduction to Mixtures - Part 1 and Part 2
*The PART 1 questions should be completed and submitted to Mr. Mak BEFORE March break.
*PART 2 questions should be done and submitted by the end of our first week back after March Break
*All students are welcome to sign out a copy of the Mixtures mini-textbook overnight, if needed.
*PART 2 questions should be done and submitted by the end of our first week back after March Break
*All students are welcome to sign out a copy of the Mixtures mini-textbook overnight, if needed.
Grade 7 - Introduction to Mixtures Assignment - Part 1
*Use the mini-textbook on Mixtures to answer each question in your Science journal. Page hints are given in brackets. Work will be checked and marked.
- LIST the first four big ideas of this unit. (page 1)
- Define volume. Include the most common units. (p. 5)
- What are two ways the amount of matter is usually measured? (p. 5)
- A long plastic cylinder, known as a _____________ in often used to measure volume. (p. 5)
- When water is placed in a graduated cylinder to measure volume, it pulls up the sides of the cylinder to make a curve. When measuring volume we use the lowest part of this curve, which is known as the ___________. (p. 6)
- Make a quick labelled sketch of the warning symbols for corrosive, poison, flammable and explosive. Briefly define each of these hazards in your own words. (p. 8)
- What are the most common units for measuring mass? (p. 9)
- Name the scientific instrument that is often used for measuring mass in a classroom. (p. 10)
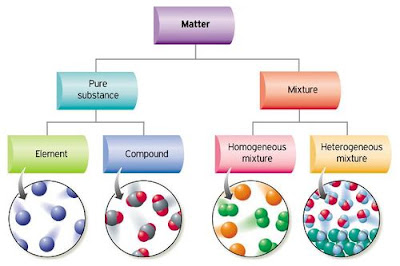
- All matter fits into one of two groups. The first group, is known as _____________. The second group is known as a _____________. (p.13)
- Explain, in your own words, the difference between a pure substance and a mixture.
- Give two real-world examples of a pure substance and a mixture. (p. 13/14/15)
- Mixtures can be subdivided into two more groups. These are: _______________ and ___________. (p. 14 - bottom)
- Define, in your own words, what a mechanical mixture is. (p. 15)
- Another name for a mechanical mixture is a _______________ mixture. (p. 15)
- Define, in your own words, what a solution is. (p. 15)
- Another name for a solution is a __________________ mixture. (p. 15)
- Give two real-world examples of mechanical mixtures (heterogeneous mixtures) . (p. 16)
- Give two real-world examples of solutions (homogeneous mixtures). (p. 16/18)
- Complete questions 2,3 on page 17.
- List three mixtures (mechanical or solutions) you have encountered in the last 24 hours. Classify each one as mechanical or solution. Have you encountered any pure substances?Matter Review Video:
Grade 7 - Introduction to Mixtures Assignment - Part 2*If needed, use the images above to help with some of the questions- List four key ideas of the particle theory of matter. (p. 34)
- Read the explore section on page 18. Explain what a solution is. (p. 18)
- Explain how a solution is different than a mechanical mixture. (p. 18)
- WITHOUT reading the information, predict whether each mixture listed at the bottom of page 18 is a mechanical mixture or solution. Next, read the text to see if your predictions are correct. (p. 18)
- Briefly explain how a centrifuge can be used to separate parts of a solution. (p. 28)
- Distillation is a method of separation that can separate a ____________ from a __________ OR a _____________ from another ____________. (p.30)
- Use the information and diagram on page 31 to explain how fractional distillation of crude oil works. (p. 31)
- List four important “fractions” that crude oil is separated into. (illustration p. 31)
- Use the glossary on p. 106-107 to define the work soluble and solubility. (p. 106-107)
- List two things that are SOLUBLE in water.
- Use the same glossary to help you explain the difference between a solvent and solute. (p. 107)
- If you are mixing “kool-aid” powder into water, which is the solute and which is the solvent? Briefly explain how you know. (p. 38)
- Explain, in your own words what dissolving means. (p. 37)
- What are three things you can do to speed up the rate at which any solute dissolves into a solvent? (p. 40 - illustration and text)
- Briefly explain how each of the methods from question #16 (stirring, increased temperature and increased surface area/smaller particles) leads to faster dissolving. (p. 40)
- Complete page 41 #2
- A concentrated solution has a lot of _________ dissolved into the _________. (p. 42)
- Define, in your own words, what a dilute solution is. (p. 42)
- Define what the word concentration means, when referring to a solution. (p. 42)
- If 75g of sugar are dissolved into 100ml of water, what is the concentration of the solution? (p. 42)
- Complete page 43 #3a/b/c (*remember that concentration should be Xgrams/100mL)
- If you are making a fruit drink by adding juice crystals to water and all the crystals dissolve, what type of solution is this? Is it saturated or unsaturated? Explain how you know. (p. 44)
- When so much solute (juice crystals) is added that it no longer dissolves in a solution with water, this is an example of a ________________ solution. (p. 44)
- Complete page 45 #2.
- The maximum solute that can dissolve in a solvent at a specific temperature is known as __________________. (p. 45)
- What are three factors that can impact the solubility of a solution? (p. 45)
- Explain, in your own words, what a supersaturated solution is. (p. 48)
- ______ is a word that refers to when a solute cannot dissolve in a solvent. (p. 49/50)Fractional Distillation Video:
https://www.youtube.com/watch?v=PYMWUz7TC3A